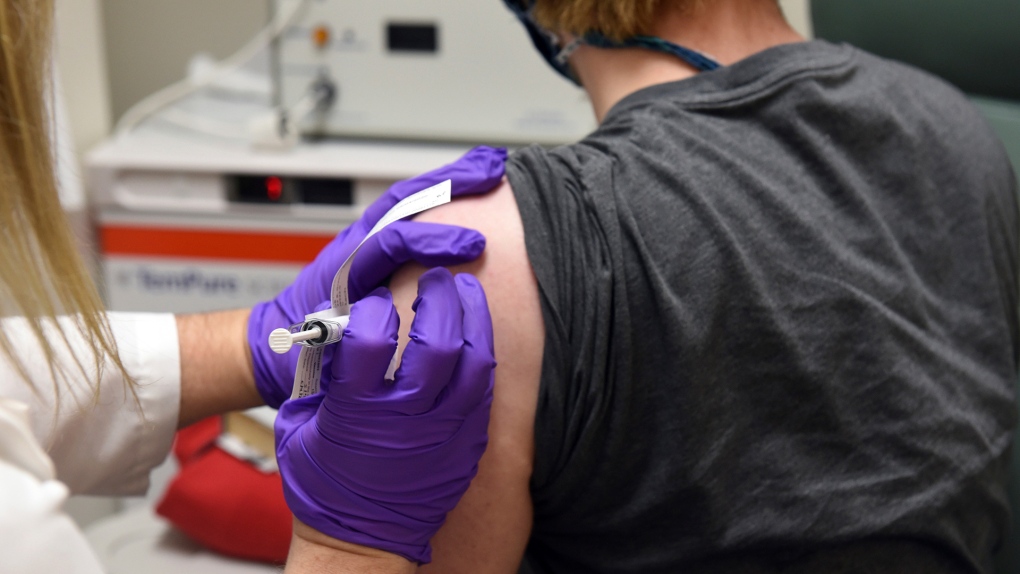
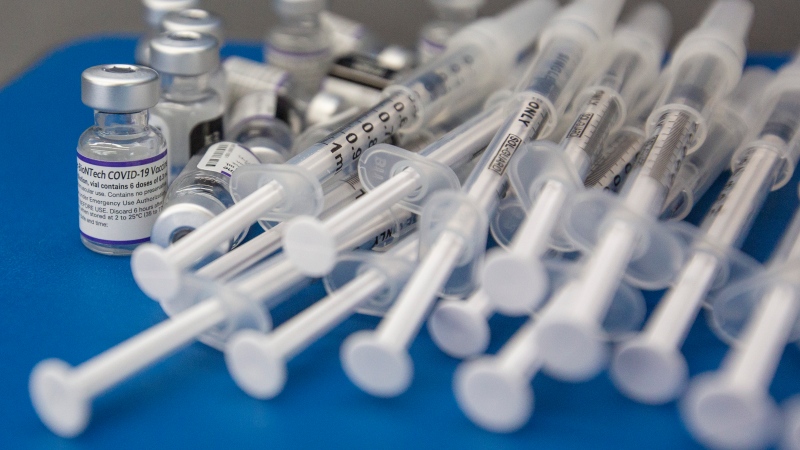
TORONTO -- Ontario expects to receive a combined 2.4 million doses of the Pfizer and Moderna COVID-19 vaccines during the first three months of 2021 with more to follow after that, Health Minister Christine Elliott says.
Pfizer said on Wednesday morning that a final analysis of the Phase 3 trial of its vaccine found it to be 95 per cent effective and that it would seek emergency use authorization from the U.S. Food and Drug Administration “within days."
The announcement comes just days after similarly encouraging news from Moderna Inc. about its vaccine. That company has said that preliminary analysis suggests that their shot is 94.5 per cent effective and is also nearing the point at which it can be submitted to the USFDA for emergency use authorization.
Health Canada will still need to approve both vaccine but work is already underway to plan for their eventual distribution.
During question period at Queen’s Park on Wednesday, Elliott said that her government is expecting to receive 1.6 million doses of the Pfizer vaccine and 800,000 doses of the Moderna vaccine in January, February and March of 2021.
Due to the fact that recipients of both vaccines will require two doses 21 days apart, it is likely that the initial shipments will only be enough to protect about 1.2 million Ontarians.
“With respect to the flu vaccine we did prioritize people in long-term care, people in hospitals and people living in congregate settings because we know that they are the most vulnerable and need to be protected. Similarly we will do the same with the COVID vaccine when it comes available,” she said. “This is major logistical challenge but we have but we have an entire group within the Ministry of Health right now planning for that, so as soon as we receive the shipments from the federal government (we will be ready).”
The Government of Canada previously signed deals with Pfizer for a minimum of 20 million doses of its vaccine candidate and Moderna for 56 million doses of its vaccine candidate. It also has deals with three other companies that are developing COVID-19 vaccines.
Speaking with CP24 on Wednesday afternoon, infectious disease specialist Dr. Abdu Sharkawy said that while the news that two of those vaccines are proving effective is positive, they won’t necessarily be approved for distribution in Canada as quickly as they may be in the U.S.
“I think the concern here is will Health Canada go along with USFDA approval? Will it accelerate things just because the USFDA has approved everything? I don’t know if that will be the case,” he said.
The shipments that the Ford government expects to receive in early 2021 will come from the four million doses of the Pfizer vaccine and two million doses of the Moderna vaccine that the federal government is expected to receive in that time period, Elliott said.